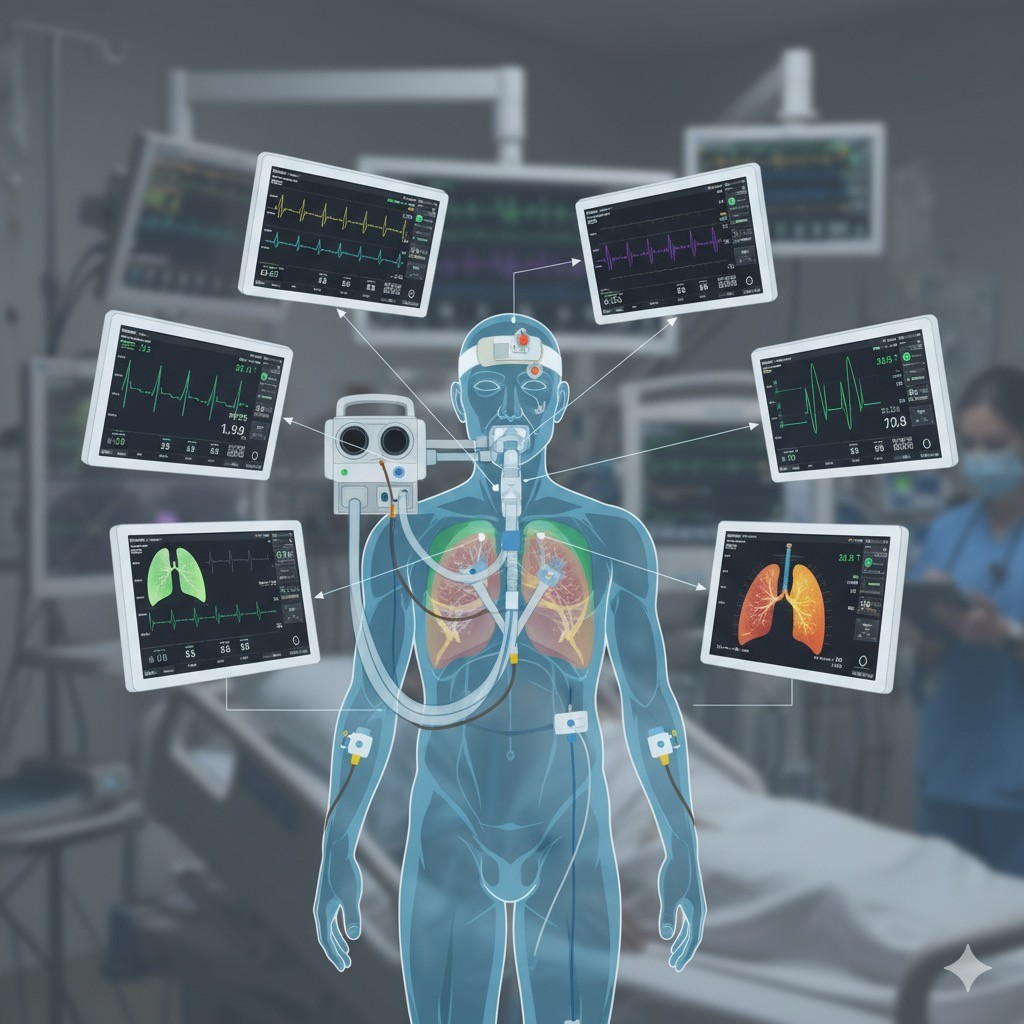
Since the inception of mechanical ventilation and for many decades we depended on numbers like tidal volumes, pressures, flow and monitoring gas exchange to monitor and treat patients on mechanical ventilators. Clearly those are not enough.
Our colleagues in the neuro intensive care have beaten us to the concept of multimodal monitoring to get a complete understanding of the brain function (blood flow, ICP, brain oxygenation, electrical status, etc.) Now it is our turn to use the same concept for patients on mechanical ventilation.
The interactions between the ventilator and the lungs, and between the ventilator and other organs, and between the lungs and other organs are very complex and our understanding of the connection pathways and ways of injury that we can create is still evolving and thus we need to look at the issue from multiple different sides and planes.
Though we are finally recognizing Ventilator Induced Lung Injury and its very harmful many negative effects, we are still scratching the surface on what cause it, how to recognize it and how to prevent it (if we ever will be that’s the question).
Goes without saying that monitoring alone even if perfect and complete (we are not there by long shot) is not enough and useless without correct interpretations and actions (the famous example is the poor Pulmonary Artery Catheter that got blamed for not improving or even worsening mortality just because it was inserted, sad story).
Anyway, let’s stick with traditional and rising nontraditional monitoring for now with the understanding that each monitoring technique has its benefits and shortcomings.
Traditional
– Ventilator: the ventilator waveforms give wealth of information about mechanics, patient ventilator interactions (PVI), measuring energy and power from the ventilator, way beyond this blog and the first line of monitoring
– Gas exchange: tell a piece of the story though shouldn’t be the only goal we seek
– Imaging: traditional imaging with x-rays, CT, US also provide a piece of the puzzle
– Hemodynamics of course goes without saying
Nontraditional
– Esophageal balloon: additional old but gold tool that gives us more information about the other side about the lung and the chest wall mechanics separately, transpulmonary pressures, setting PEEP, PVI, etc. Its use is very slowly rising but in personal opinion should be a routine monitoring tool in my opinion
– EIT: rising technology that is actually pretty old from the 70s but finally making its presence and benefits felt at the bedside from measuring volume, blood flow distribution and thus V/Q would be an integral part of future monitoring.
– Volumetric capnometry: another oldy but goody that adds lots of information like dead space, VCO2, and again should be a routine monitoring technique.
– FRC: some ventilators are capable of measuring the FRC which might be helpful as a monitoring technique.
– Evolving technologies (measurements of stress, strain, tidal volume distribution, etc.)
– AI driven closed-loop systems, love it or hate it, its reality and will be there sooner than you think. It will be capable of better instantaneous interpretation of data from different monitoring and immediate action (if you ask me, I love it).
Think of the whole process as a symphony, the clinician is the conductor and all the monitoring are the musicians that play different tunes and have to work in harmony to produce a beautiful music piece. So how we will be better conductors and incorporate and use all the available information from the existing and the new coming ones to improve our outcomes will be challenge but we can do it friends.